How fungi elude antifungal treatments
Article Highlights
- Research led by Tuo Wang of Michigan State University has shown how fungi reinforce the cell wall after exposure to antifungal drugs.
- Fungal infections, which affect more than 2 million people around the world every year, are difficult to treat because fungi develop resistance to the four families of drugs available on the market.
- The results of Wang and his team, published in the journal Nature Communications, could help researchers develop new treatments to address fungal infections in humans.
Every year, life-threating invasive fungal infections afflict more than 2 million individuals globally. Mortality rates for these infections are high, even when patients receive treatment.
Aspergillus fumigatus, the most frequent cause of invasive fungal infection in people with suppressed immune systems, is responsible for approximately 100,000 deaths annually around the world. Poor treatment outcomes result from therapeutic failures and the fungi’s resistance to existing drugs.

A new multi-institutional study led by researchers at Michigan State University has characterized how fungi adapt to restructure their cell walls, effectively thwarting current antifungal medications. This new information opens opportunities to devise more effective use of antifungal drugs. The results were published July 31 in the journal Nature Communications.
“In order to improve the use of and develop new antifungal drugs, we need to understand the target,” said Tuo Wang, the inaugural Carl H. Brubaker Jr. Endowed Associate Professor in the Department of Chemistry at Michigan State University and lead author on the study. “This is not done easily, because the cell wall is very complex.”
The study was also selected to be featured among the journal's Editors' Highlights as one of the 50 best papers Nature Communications has published recently in the area of Microbiology and Infectious Diseases.
With this work, Wang and his team believe they have laid the foundation for pharmaceutical companies to adapt or combine existing antifungal drugs to help overcome their previous limitations.
Cellular remodeling
Antifungal drugs target molecules in the fungal cell wall, a flexible, but rigid outer layer that provides the cell protection. By rupturing the protective structure, the drugs kill the fungal cell to control the fungal infection.
One of the newest families of antifungal drugs, echinocandins, target essential building blocks in the cell wall known as ß-glucans. This attack should be effective, but fungi are extraordinary organisms that have evolved survival strategies to rebuild and reinforce the wall’s architecture.
In their new report, Wang and his colleagues determined the atom-by-atom configuration of the cell wall after exposure to echinocandins. To do this, they used biochemical analysis and state-of-the art imaging techniques, including solid-state nuclear magnetic resonance, dynamic nuclear polarization, transmission electron microscope and atomic force microscopy.
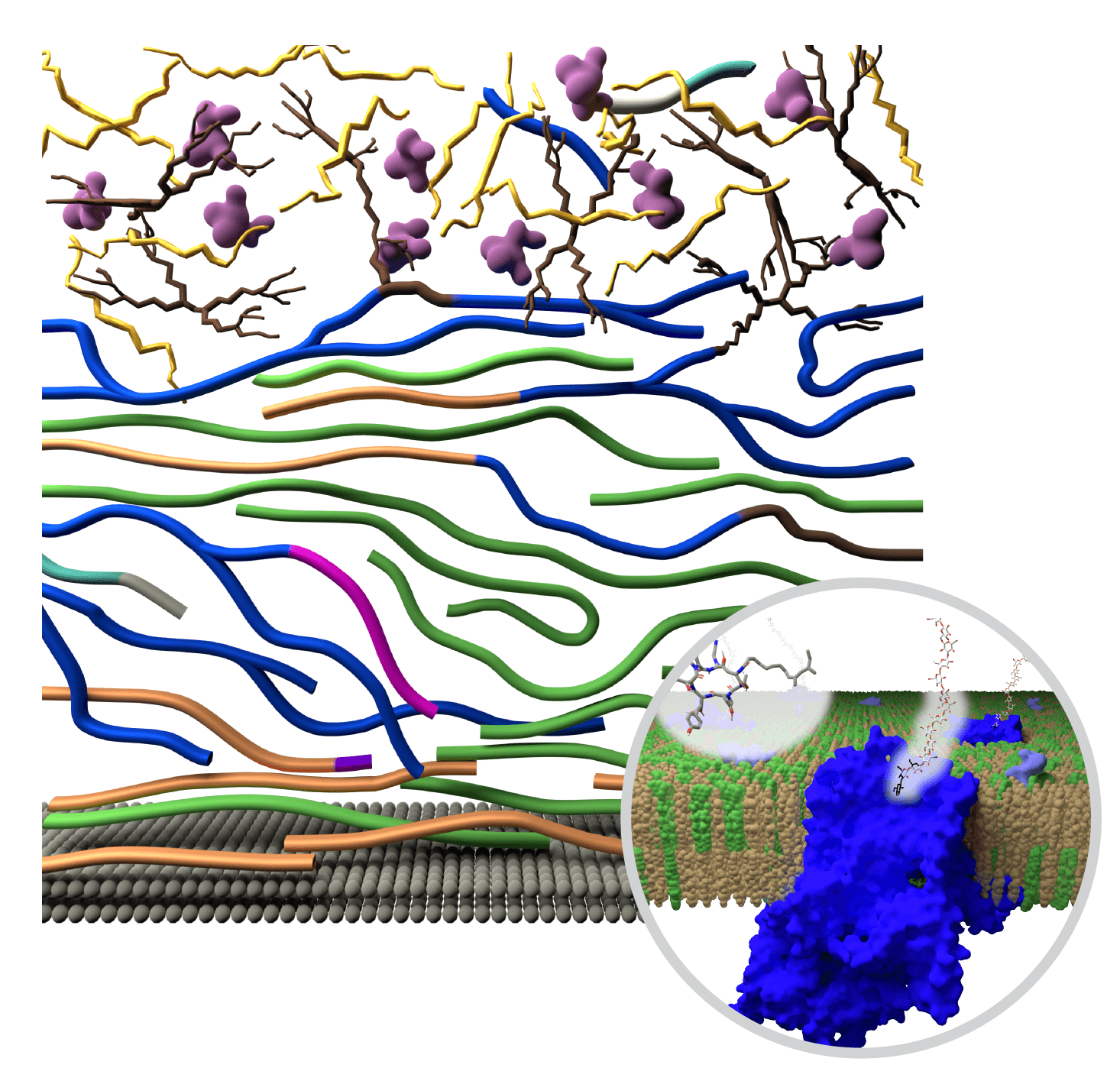
They then shared the results with a team at the MSU-Department of Energy Plant Research Laboratory, or PRL. The PRL team developed molecular dynamic simulations to illustrate nanoscale changes that unfold over hours to days in the fungal cell wall.
“NMR tells us that things are reacting but there is no picture,” said Josh Vermaas, assistant professor in the PRL. He’s also affiliated with the MSU Department of Biochemistry and Molecular Biology and the Molecular Plant Sciences Program.
Vermaas is a co-author on the study, who, together with Daipayan Sarkar, a research associate in the PRL, conducted the simulations portion of the study.
“We created visually appealing pictures of how molecules come together at the nanoscale, simulating the molecular details that we otherwise wouldn’t get access to,” Vermaas said.
The team found that when exposed to echinocandin, the fungi enhance their survival odds by making specific changes to the structure and organization of the components in their cell walls. In particular, as the concentration of ß-glucans goes down, fungi rapidly increase the presence of different, but related molecules to regenerate and preserve the integrity of the cell wall.
In addition, polysaccharide structures, such as galactomannan and galactosaminogalactan, are reshuffled to enhance the stiffness and hydrophobic nature of the polymer network in the membrane.
“We found the supramolecular assembly has been fully reshuffled,” said Wang. “This dynamic dance unfolds both at the chemical and nanoscale levels, rendering the cell wall sturdier yet pliable, ensuring survival under stress.”
In this simulation created by the Vermaas lab, small molecules, identified by imaging techniques, come together in the cell wall to reinforce the protective structure when exposed to fungicides. Credit: A simulation adapted from Dickwella Widange et al. Nat. Commun. (2024).
Not only did the fungal response to the drug increase the strength and resilience of the cell wall, the new architecture also eliminates the drug target in many instances. This renders the drugs ineffective against fungal spread.
“Biology is wild,” said Vermaas. “Evolutionary pressure allowed for these kinds of mechanisms to develop, but holy moly. How did fungi figure this out?”
Fungal spores are ubiquitous in the environment, but a healthy person’s immune system can sweep the spores from the body. People with compromised immune systems, however, are susceptible to the spores taking hold. That means, for example, people undergoing cancer treatments, receiving organ transplants or fighting other diseases, including AIDS and COVID, will have a harder time clearing the intruders.
In the body, fungi become established in the lungs and send long, branching structures called hyphae deep into lung tissue. Although drugs or surgery can alleviate an infection, once it’s in place, it is almost impossible to eliminate.
Only four families of an antifungal drugs are currently on the market, each limited by the evolutionary fungal roadblocks, such as the one identified in this study. That’s why the availability of effective antifungal drugs is needed now more than ever, Wang said
“We are doing the fundamental science,” said Wang. “Now that we understand how fungi survive antifungal treatment, this knowledge will be helpful for the development of new drugs.”
Also contributing to the study were Isha Gautam and Shi-You Ding at MSU; Frederic Mentink-Vigier at the National High Magnetic Field Laboratory; Andrew Lipton at the Pacific Northwest National Laboratory, Thierry Fontaine at Paris Cité University; Jean-Paul Latgé at the University of Crete and Ping Wang at Louisiana State University Health Sciences Center.
This work was funded in part by the U.S. Department of Energy, Office of Basic Energy Sciences (grant number DE-FG02-91ER20021).
- Categories: