How viruses know what’s theirs

Dr. Sundharraman Subramanian (left) and Professor Kristin Parent alongside one of MSU’s new cryo-electron microscopes. The blue light indicates the instrument is operating at correct cryo-genic temperatures. Credit: Paul Henderson / College of Natural Science
Why this matters
- While replicating in a host cell, viruses package their genetic material with near-perfect accuracy into protein shells called capsids. Capsids act like molecular armor, protecting a virus’s genetic cargo.
- Understanding how viruses are so efficient at this packaging could help researchers create their own lab-made versions of capsids with similar traits
- These synthetic capsids could carry helpful genetic cargo used in gene therapy, new antivirals, and a range RNA-based therapeutics.
EAST LANSING, Mich. — Researchers at San Diego State University and Michigan State University are shedding
new light on how viruses meticulously pack their genetic material — a breakthrough that could help researchers engineer antivirals and gene therapies.
The team’s findings, which are published in the Proceedings of the National Academy of Science, reveal how a combination of molecular properties allow viruses to selectively gather their own RNA into protein shells called capsids while ignoring a host cell’s own competing genome.
Knowing how viruses package their RNA with high selectivity — a feat achieved with more than 99% accuracy by some viruses — could help scientists engineer synthetic capsids with similar traits and leverage them as powerful scientific tools.

The new instruments at MSU’s cryo-EM facility are able to capture 10 times as much data in a 24-hour period. This increased data-collection means researchers can observe especially rare, microscopic events. Credit: Paul Henderson / College of Natural Science
This research was achieved by combining next-generation sequencing technology with newly installed instruments at MSU’s cryo-electron microscopy facility, as well as support from the National Institute of General Medical Sciences and the National Science Foundation.
“From a health perspective, synthetic capsids can be used to create antivirals that target RNA packaging which can impact humans, plant and animal agriculture, as well as veterinary medicine,” Kristin Parent said, director of MSU’s cryo-EM facility and an author of the latest paper.
The latest breakthrough was the result of a collaboration between Spartan researchers and those in the Garmann laboratory at San Diego State University, which examines the complex molecular choreography behind viral replication, infection, and evolution.
“Some RNA viruses are built from fewer than 200 molecules,” Rees Garmann said, an assistant professor in SDSU’s Department of Chemistry and Biochemistry and senior author of the new study. “And yet they are able to accomplish remarkable feats, like replicating in astronomical numbers and building precise nanoscale structures.”
The findings, which shed new light on longstanding questions in virology, further benefited from the timing of a $20 million expansion to MSU’s cryo-EM facility.
The team’s research is some of the first to utilize the suite of newly installed instruments, which were secured with the help of MSU’s Office of Research and Innovation, the Office of the Provost, and the colleges of Natural Science, Human Medicine, Osteopathic Medicine and Engineering.
“The expansion of MSU’s cryo-EM facility with state-of-the-art instrumentation allows researchers across many disciplines to investigate how biological structure at the molecular level controls function,” Doug Gage said, MSU’s Vice President for Research and Innovation.
“We will be able to answer questions that were once beyond our reach.”
After flash-freezing biological molecules, cryo-EM takes thousands of pictures to build detailed 3D models that researchers use to understand the microscopic mechanisms of life.
“The two microscopes we’ve added mean that for the first time, we have the latest and greatest cryo-EM technology at our fingertips,” Dr. Sundharraman Subramanian said, cryo-EM facility manager and a study co-author.
“What’s most exciting is our ability to collaborate with researchers on and off campus, to work on new ideas, and being able to assist scientific discovery with the tools we now have.”
The host with the most
To illustrate the staggering quantities of viruses found on our planet, Parent will sometimes offer her students an eye-popping figure: if you scoop up two handfuls of water from Lake Michigan, you’d be holding more viruses than there are humans on Earth.
Among these viruses, the most abundant types are bacteriophages, or phages — viruses that infect and replicate within bacteria. In their new study, the researchers examined a phage called MS2, which preys on E. coli.
Viruses rely on the molecular machinery of other cells to replicate. When MS2 attaches to a bacterium, it injects its own genetic material, forcing the host cell to assemble viral copies.
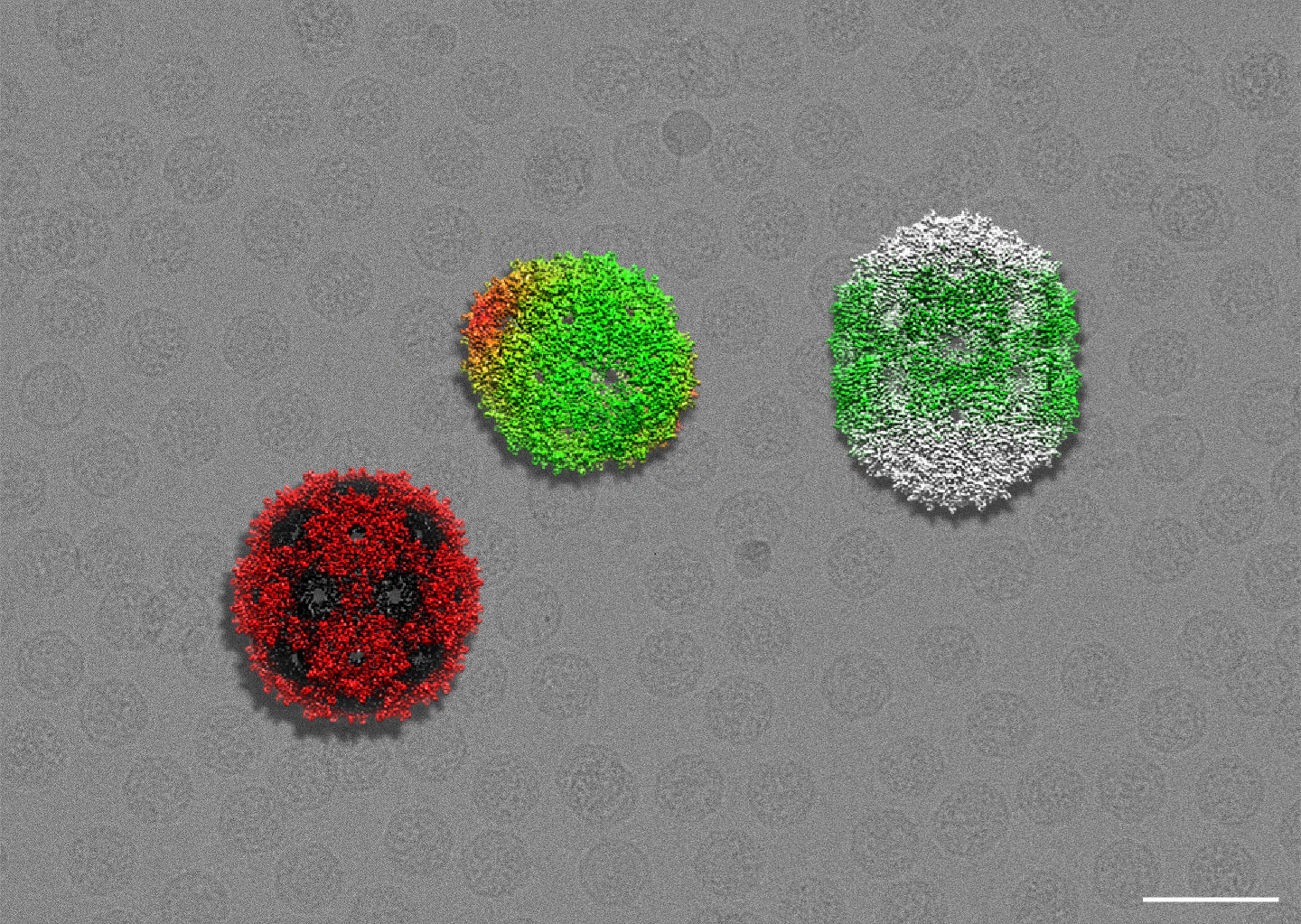
By scrambling MS2’s genome, the researchers were able to observe a variety of viral packaging outcomes. The red and black particle on the left represents a correctly formed capsid, while the capsids to the right display mistakes brought on by changes to their RNA. The gray background is a cryo micrograph, showing hundreds of phages. Credit: Kristin Parent
During this process, viral coat proteins assemble around viral RNA to form a capsid, which protects the genetic cargo. With 180 identical coat proteins arranged to make 20 different sides, the resulting virus looks a bit like a soccer ball or a game die.
Eventually, when the host cell bursts open, a new generation of these phage copies is released.
For researchers like Garmann and Parent, the question was how the phage can recognize its own genome and package it so efficiently, especially when the RNA is mingling with the host’s competing genetic material inside the cell.
“Around 99% of the particles we’re seeing at the end are perfectly formed viral copies, so it’s a high-fidelity process,” Parent said, who’s a professor in MSU’s Department of Biochemistry and Molecular Biology.
RNA origami
Compared to DNA’s iconic double-helix, RNA is single-stranded. This means it can form complex secondary structures like bulges, loops, and hairpins.
Previously, researchers thought a particular structure called a TR stem-loop acted as a packaging signal for MS2. You might think of this as a molecular signpost indicating where viral RNA packaging should begin.

To see what other factors might influence packaging, the researchers systematically scrambled the MS2 genome, producing RNA constructs with unique properties. These included molecules of varying shape, length and sequence. Some of these molecules also had stem-loop structures placed at certain locations or lacked them altogether.
Like watching finished products roll off an assembly line after major changes on the factory floor, the team then analyzed capsid packaging outcomes to determine the impact of these RNA-tweaks.
MSU’s newly expanded cryo-EM facility helped the researchers glimpse microscopic events that previous instruments would’ve otherwise missed.
Specifically, they were able to see unique and often surprising capsid packaging results — viral particles that were too small, and even those with inefficient shapes. Glimpsing these provided clear hints as to which RNA properties were necessary for perfect packaging.
“We no longer have a bottleneck when it comes to how many images we can take, and so we’re able to identify far rarer events,” Parent said, who explained that the new instruments allow them to collect ten times more data in a 24-hour window.
“These instruments also allow researchers to study different sample types, like tissue samples and thicker cell samples,” Subramanian added.

What the researchers ultimately discovered was that MS2 coat proteins on their own are highly capable of selectively packaging viral RNA, and that a diverse group of RNA properties, not just the well-known TR stem-loop, had an outsized impact on the process. This included RNA length, sequence and various stem-and-loop structures making a collective difference.
Through their findings, the team is helping rewrite our understanding of how some viruses achieve their impressive RNA-packaging feats.
With synthetic capsids and new genetic cargo, these same molecular mechanisms can be leveraged for the greater good — from gene editing and vaccines to the next generation of RNA-based therapeutics.
“Not only does this work hold promise for applications in targeted drug delivery and gene therapy, but it also advances our fundamental understanding of how molecular mechanisms function to make viruses work so efficiently,” said Amineh Rastandeh, a Garmann lab graduate student and lead author of the study.
- Categories: