Radical new chemistry from MSU
There’s a scene in the 1992 movie “A League of Their Own” in which Tom Hanks’ character says, “It’s supposed to be hard. If it wasn’t hard, everyone would do it.”
He’s talking about baseball, but a similar sentiment applies to synthetic chemistry: Doing either at the highest level is no easy task.

Selvan Demir, assistant professor, MSU Department of Chemistry. Courtesy photo
That’s especially evident in recent research from a team of Michigan State University chemists who have unveiled a new class of magnetic molecules.
Reporting in the Journal of the American Chemical Society, researchers led by Selvan Demir have brought together famously challenging building blocks to push single-molecule magnets a step closer to their promising applications.
“Realizing these molecules in a laboratory setting is extremely difficult,” said Demir, an assistant professor in the Department of Chemistry in the College of Natural Science. “But this challenge has been met by Spartan chemists for the first time.”
These single molecules work like ultra tiny bar magnets and were first synthesized in the 1990s. Since then, researchers have been developing new types with the goal of pushing established magnetic technology, including hard drives, to a whole new level. Single-molecule magnets also could open new doors in emerging technologies, such as quantum computers.
Getting to those applications, though, still requires fundamental innovations. Demir and her team have a history of demonstrating the ingenuity and persistence needed to deliver those.
A lanthanide and a bismuth radical walk into a bar magnet
On paper, the approach Demir and her team took makes a ton of sense.
The electrons zipping around the exterior of atoms have something called spin. It’s a fundamental physical property of tiny particles that researchers can take advantage of for certain applications, similar in that regard to electric charge.
While charge gives rise to electricity, spin is more connected to other things, including magnetism.
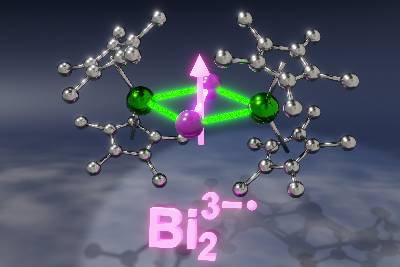
The new single-molecule magnet produced by Michigan State University chemists. The bismuth radical anion, shown in pink and labeled Bi23−•, bridges two lanthanide ions shown in green. Credit: Demir Group
In building their new single-molecule magnets, Demir and her team wanted to combine different atomic building blocks whose electron spins could interact or couple in a way that would yield molecules with tantalizing magnetic properties.
So the team picked two building blocks that could couple spins in an unprecedented way. But these choices still had their challenges.
The first key component are lanthanides, which are large, unwieldy elements that live at the bottom of the periodic table. The other is what’s known as a bismuth radical anion.
It’s the “radical anion” part that makes it particularly challenging. That means it’s a version of bismuth that carries a negative charge and is typically very reactive and short-lived.
The team’s earlier work had hinted that combining the ingredients could be fertile ground for making single-molecule magnets, but progress was hard won because making the molecules presented obstacles in virtually every phase: synthesis, isolation and purification.
Still, Demir knew that these cantankerous components could actually work together.
“An elegant way to stabilize a radical anion is to pair it with lanthanide ions,” Demir said. “And placing a bismuth radical in between lanthanides would result in effective magnetic communication between the species.”
Capitalizing on its experience, Demir’s team realized this synergy and overcame the hurdles presented by the individual pieces with careful design and judicious planning, she said.
“The new molecules constitute the first single-molecule magnets containing bismuth radicals,” Demir said. “This is also the first time that a bismuth radical was isolated with any lanthanide or transition metal from the periodic table.”
Also joining the MSU researchers on this project were chemists led by Nicholas F. Chilton, a professor at the University of Manchester in England. Chilton’s team performed computations to help better understand the electronic and magnetic properties of the new molecules.
Although more research and development will be required to bring these molecules to commercial technologies, this discovery creates new opportunities to reach that goal.Banner image: Using notoriously challenging ingredients, Michigan State University chemists have created single-molecule magnets that could enable new data storage and computational technology. Credit: Demir Group