Life without lipids: Building toward 'artificial cells'
Article Highlights
- Researchers from Michigan State University, Lawrence Berkeley National Laboratory, Pennsylvania State University and the University of Delaware teamed up to create a new way to design cell-sized reactors.
- Drawing inspiration from bacteria and forces at work in the biological world, the team created protein-coated shells that can be packed with active cargo including enzymes and RNA.
- Published in the journal Small, these “artificial cells” could open new paths to myriad useful things, including medicines, biofuels and a deeper understanding of fundamental biological principles.
In a bit of an ironic twist, research supported by the National Science Foundation’s Big Idea program recently appeared in the journal Small.
But it’s fitting, considering the journal features breakthroughs in microscopic and nanoscale science. And the nationwide team supported by the NSF was tackling a challenge to help reveal the rules of life.
“The grant asked the intriguing question, ‘What if Life evolved without lipid membranes?’” said Eric Young, a postdoctoral research scientist on the research team. He works in Cheryl Kerfeld’s laboratory, which operates at both Michigan State University and Lawrence Berkeley National Laboratory.
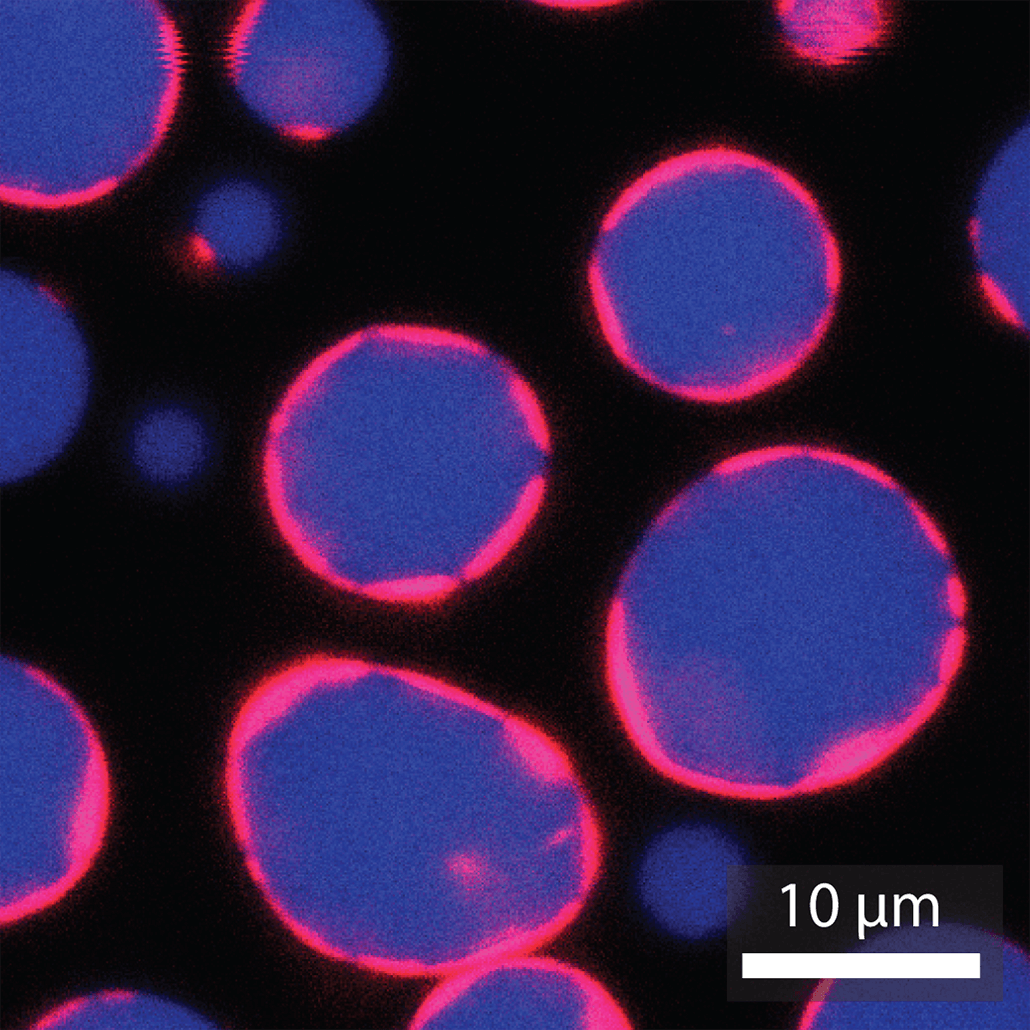
Every living cell is enveloped in a bubble-like membrane made from fatty building blocks known as lipids.
But Young, Kerfeld and their colleagues at Pennsylvania State University and the University of Delaware aren’t interested in replicating life as much as they are in borrowing and exploring themes from it.
“The motivation for this paper comes from a larger collaboration called the ‘ProteoCell’ Project,” said Dharani Abeysinghe, a doctoral student at Penn State and co-lead author of the new report.
That project includes the three research groups behind the Small publication, along with collaborators at Arizona State University, the University of Minnesota and the University of California, Santa Barbara.
“The goal there is to build a synthetic cell without the use of lipids,” Abeysinghe said. “We also call it the fat-free cell.”
For the Small project, the team took a step toward that by engineering simpler, cell-like factories that could one day produce myriad useful things, including medicines, biofuels and a deeper understanding of fundamental biological principles.
The field of biotechnology already enlists microbes such as yeast and E. coli to produce therapeutics. When it comes time to purify these valuable products, though, membrane lipids are one of the most problematic contaminants, the researchers said.
So the researchers reasoned that they could forge a new path to these applications by creating designer membranes from materials other than lipids. With its Small publication, the team has provided a proof-of-concept for that hypothesis.
“It worked well,” Young said. “We still need to learn more as far as the specifics of the membranes, but this was a great first pass.”
Supersized compartments
At MSU, Kerfeld is a Hannah Distinguished Professor in the Department of Biochemistry and Molecular Biology and a member of the MSU-Department of Energy Plant Research Laboratory. She has extensive experience working with what are known as bacterial microcompartments, or BMCs.
These tiny compartments operate as organelles inside bacteria, where they use protein shells to isolate their inner workings from their surroundings. The shells typically measure a few dozen to a couple hundred nanometers across, or roughly 1 to 10% the size of a cell.
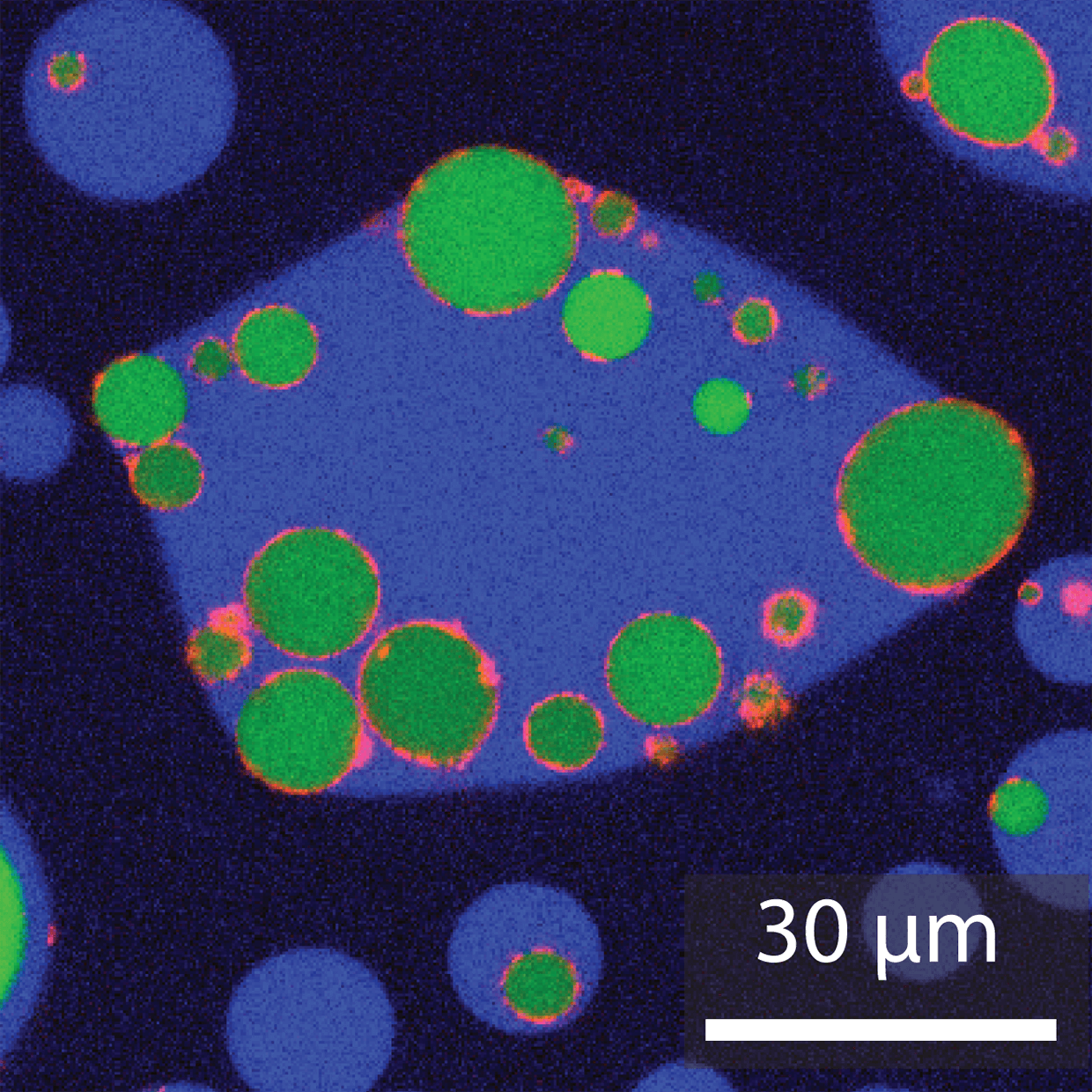
With support from the NSF, Kerfeld, Young and their colleagues have now used these proteins from BMC shells to create much larger compartments. Rather than a fraction of the size of a cell, the new structures are the size of cells themselves.
“These much larger shell structures are more amenable to bona fide, complex cell-like behaviors or ‘living’ applications rather than just housing a specific pathway at a subcellular scale,” Young said.
Christine Keating, the Shapiro Professor of Chemistry at Penn State, led the team’s Happy Valley cohort. Millicent Sullivan, the Alvin B. and Julie O. Stiles Professor and Chair of the Department of Chemical and Biomolecular Engineering, was the team leader at University of Delaware.
A key component of the work was taking advantage of what’s called liquid-liquid phase separation, which is the same thing that drives oil to separate from water. Phase separation is common inside living cells and also happens to be a specialty of Keating’s lab at Penn State.
The team mixed together bio-friendly polymer solutions that would phase separate, forming droplets. These droplets then provided cell-sized templates for the proteins to coat and create compartments that, going forward, can be packaged with various types of active biomolecules.
"With our initial experiments showing we can load four different types of cargo and contain an active coupled enzyme reaction,” Young said. “We’re excited to prototype different cargos that cater to medicine, bioenergy and space applications."
The researchers can also tinker with the protein composition of the enclosures to give them different functionalities, such as behaving as scaffolds or catalyzing electronic reactions, Abeysinghe said.
"Not only do we have a customizable container with regard to what goes inside, in the future, we could change how the molecules pass through the shell or engineer specific binding sites for cargo,"Abeysinghe said, "We imagine scientists and engineers could use this system for ‘plug-and-play’ reactions for what they desire eventually."
The team is also eager to do further experiments to characterize the shells they’ve created and learn how to control various parameters, such as their size, shape and thickness. In the meantime, they’re excited that their big idea worked.
“The work is special for it’s ‘new to nature’ results,” Kerfeld said. “Eric and Dharani came together from two different disciplines, each brought their own specialty building material and they combined them, to get something entirely new that isn’t likely to emerge from evolutionary tinkering.”