Ask the expert: How — and why — do you harvest isotopes?
Article Highlights
- Katharina Domnanich is an assistant professor working at the forefront of discovery at the Facility for Rare Isotope Beams, or FRIB.
- While FRIB creates isotopes the world has never seen before, it will also create isotopes that researchers are already studying for applications in medicine, plant science and more.
- Domnanich is helping build a laboratory that will help accelerate those studies by “harvesting” those isotopes and, while doing so, she’s also exploring new research areas to help support FRIB’s mission.
Katharina Domnanich joined Michigan State University in 2018, when the Facility for Rare Isotope Beams, or FRIB, was entering its final phases of construction.
The facility was closing in on becoming a world-class particle accelerator and user facility for the U.S. Department of Energy Office of Science, or DOE-SC, making short-lived isotopes that couldn’t be made anywhere else.
These exotic isotopes will help us better understand fundamental rules of nature and find answers about how the early universe was formed. But Domnanich wasn’t most interested in these fast-decaying nuclei that have likely never existed on Earth before.

Rather, she was drawn to the more common isotopes that researchers already knew could be useful for a variety of applications that would be made in the background of FRIB’s isotope discovery. Domnanich came to FRIB to work as a postdoc with Gregory Severin, associate professor of chemistry at FRIB and in the MSU Department of Chemistry, who was building what’s called an isotope harvesting laboratory.
Severin’s team was assembling technology to extract or harvest those “by-product” isotopes and make them available to other researchers who could put them to work in fields like medicine, plant science and many more. The isotopes are harvested during routine operation for FRIB’s nuclear physics mission — without interfering with its primary users. The U.S. Department of Energy Isotope R&D and Production Program, or the DOE Isotope Program, supports isotope harvesting at FRIB.
Today, FRIB is officially up and running and its isotope harvesting laboratory is nearing completion. Domnanich is now an assistant professor of chemistry at FRIB and in the MSU Department of Chemistry who has started her own team, won a 2023 FRIB Achievement Award for Early Career Researchers, and branched out into new research areas.
In fact, she recently published about one of those in the journal Applied Radiation and Isotopes.
But she’s still a driving force on the team making isotope harvesting at FRIB a reality, working alongside Severin and their colleagues.
The College of Natural Science caught up with Domnanich to talk about the project, as well as what it was like launching her career at FRIB and what’s on the horizon for this rising star of nuclear science.
This conversation has been edited for length and clarity.
You joined FRIB to work on isotope harvesting, yet your new paper is about something else. It sounds like there’s no shortage of things to work on at FRIB.
Oh no. There are tons of things to do here.
My group is working on isotope harvesting, and I have newer projects working on what’s called mass separation. These all use radioactive isotopes and, to work safely with those, experiments have to be like a well-rehearsed show.
To prepare students, they have to do an experiment at least 10 times with nonradioactive materials before using the radioactive stuff so they know how to handle everything.
So, in addition to all the work we have to do, students also have a lot of pre-experiments. If you ask them, they’ll tell you we’re always busy.

Why did you decide to stay at MSU and FRIB after completing your postdoctoral research?
I really enjoyed working together with Greg. It was incredible and a very productive time. I liked the dynamics in the group and how isotope harvesting at FRIB was being developed, so when there was an opening, it just seemed like a great opportunity.
You were recognized with an FRIB Achievement Award for Early Career Researchers in 2023 — the year after you became a faculty member. What was that like?
It felt very encouraging. I was very positively surprised that I got that award, and it was really nice.
It also feels like there is a lot of interest in isotope harvesting and that people really want to use the isotopes. I know there are organic chemistry professors who are interested and some plant biologists. We also have a Department of Radiology where people are interested.
So, I think there will be tons of opportunities for collaboration when the isotope harvesting is running.
What sorts of things do researchers want to do with isotopes harvested from FRIB?
A lot of the isotopes are for nuclear medicine. For example, we’ve worked with scandium-47, which is being studied as a therapeutic for cancer treatment.
When I was a postdoc, I also looked into collecting zinc-62, which is interesting for nuclear medicine and also for plant sciences. Plants needs zinc, and I showed that plants could take up zinc-62, then I scanned the plants to see where it goes. That could be quite a nice tool to study plant systems.
Then for materials science, you can use certain isotopes to visualize leaks and cracks in pipes and things like that.
We could also harvest isotopes to be used in nuclear batteries. The rovers we send to Mars use radioactive isotopes in their batteries, and some of those same isotopes will be produced at FRIB.
So, how do you harvest isotopes?
At FRIB, we accelerate ions into a primary beam that reacts with a target and produces secondary beams. It’s these exotic secondary beams that are used by nuclear physicists and nuclear scientists to study reactions that happen in stars, for example — or whatever they want to explore.
But maybe just 20% of the primary beam goes into producing secondary beams. That means you have 80% of the primary beam that you still need to do something with. It’s usually stopped by a solid metal block, just to have something that can absorb all its energy.
At FRIB, we want to instead stop it inside a rotating drum of water. By stopping the beam within water, you have tons of reactions happening between the beam particles and water molecules, producing many new isotopes in the process.
Those isotopes are then just floating in this water system. And FRIB’s water system will be huge, like 7,000 liters or almost 2,000 gallons as a ballpark number.
We can capture the isotopes on ion exchange resins, which function like a really fancy Brita filter. From there, we can extract them and purify them, thereby making them available for further experiments.
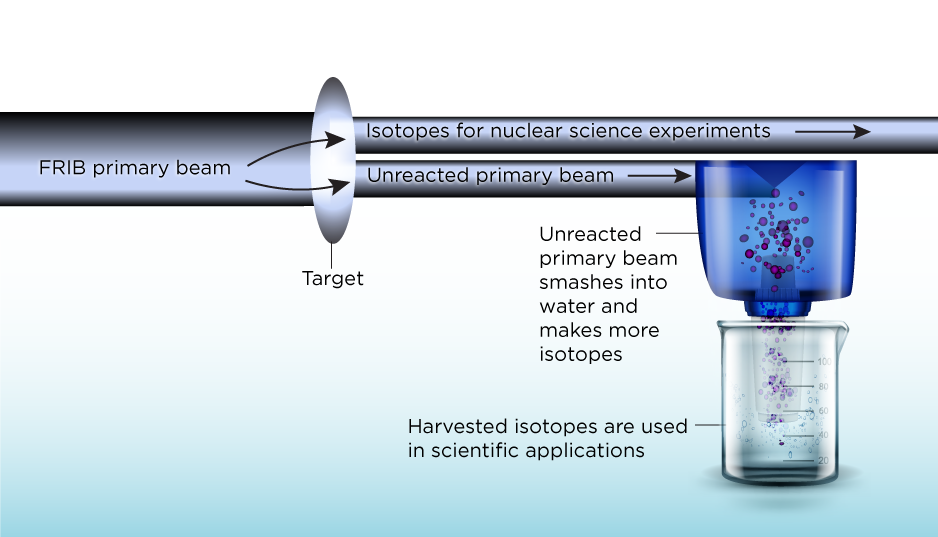
The Facility for Rare Isotope Beams is building a new lab that will use ions in FRIB’s unreacted primary beam to create isotopes that can be harvested for a variety of applications. Image courtesy of FRIB
What’s the status of the isotope harvesting lab now?
We did some proof-of-principle tests when I was a postdoc in Greg’s group with a smaller type of water system. So that had about 50 liters of water instead of 7,000. But even that helped us develop the chemistry and learn what the reaction rates are for certain isotopes. Still, scaling up is a huge task.
While you’re bringing the isotope harvesting lab online, you’re also exploring some new directions in your group, which led to your recent paper. Can you talk more about that?
My group is looking into something called mass separation to purify isotopes, and that’s the connection to the new paper.
What is mass separation?
Remember that I said we use something like fancy Brita filters in isotope harvesting? If you have a fancy setup, like us, you can separate the individual elements by their chemistry. You can separate the sodium from the magnesium from the calcium and so on.
But with a filter, you can’t separate different isotopes of the same element. Calcium, for example, has several stable isotopes, like calcium-40, calcium-42 and so on. That makes it almost impossible to separate isotopes using chemistry.
But mass separation is feasible. These isotopes all have different masses, and you can separate them by their mass using an incredibly strong magnet.
And how does that fit in with your new work?
I was working with another group led by Georg Bollen, director of the Experimental Systems Division at FRIB, and they have a setup to generate so-called offline beams.
Before the main FRIB accelerator was running, but after FRIB’s predecessor, the National Superconducting Cyclotron Laboratory, was already switched off, researchers needed to use something else to be able to study rare isotopes. That’s why they made the Batch Mode Ion Source, or BMIS.
It’s a device that can make a lower energy beam than FRIB’s main beam, but it needs to have source samples that have specific chemical and physical properties. I collaborated with this BMIS group to prepare those source samples.
Actually, our last paper is about the preparation of source samples. And that fits into the mass separation project because I want to use this ion source and magnet as part of the mass separation setup to do my next mass separation experiments.
So this was very good preparation for me, kind of like training, to figure out what is necessary to establish mass separation for further experiments.
Last question: Do you have a favorite isotope?
Yes. I love scandium-43.
It’s a rare-earth-like element, which means it’s useful in electronics, but it also has cool applications in nuclear medicine. You can use different isotopes of scandium for therapy and diagnostics, so you can think about using the same medicine for diagnosing cancer and for treatment.
I worked with scandium a lot during my doctorate — I spent so many hours working with different scandium isotopes in the lab — and I still really like it.
Michigan State University operates the Facility for Rare Isotope Beams (FRIB) as a user facility for the U.S. Department of Energy Office of Science (DOE-SC), supporting the mission of the DOE-SC Office of Nuclear Physics. Hosting what is designed to be the most powerful heavy-ion accelerator, FRIB enables scientists to make discoveries about the properties of rare isotopes in order to better understand the physics of nuclei, nuclear astrophysics, fundamental interactions, and applications for society, including in medicine, homeland security, and industry.
The U.S. Department of Energy Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of today’s most pressing challenges. For more information, visit energy.gov/science.
The U.S. Department of Energy Isotope R&D and Production Program (DOE Isotope Program) supports isotope harvesting at FRIB. MSU operates FRIB as a user facility for the Office of Nuclear Physics in the U.S. Department of Energy Office of Science, supporting the mission of the DOE-SC Office of Nuclear Physics.