Complex biological phenomenon may have a surprisingly simple explanation
Life is messy, even at microscopic and molecular level, but Michigan State University researchers have shown that some straightforward science can still account for important biological behavior.
Reporting in the journal eLife, the Spartan team led by Michael Feig and Lisa Lapidus showed that relatively simple characteristics help RNA and proteins organize themselves. Researchers believe that when these biomolecules congregate, or condense, it can help speed up or enhance a range of cellular functions.
“The main consequence of such condensates is that it may bring functionally related biomolecules closer together,” said Feig, a professor in the Department of Biochemistry and Molecular Biology in the MSU College of Natural Science (NatSci).
“Having them condensed could speed up the process because you don’t have to wait for a molecule to show up from far away,” said Lapidus, a professor in the MSU NatSci Department of Physics and Astronomy.
Although more research is needed to reveal the exact workings of condensates, the MSU team has revealed that relatively basic traits of the biomolecules involved can spur what’s known as phase separation.
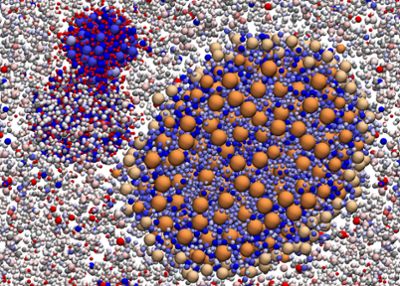
Specifically, when RNA and proteins are large enough and have sufficiently strong and opposite electric charges, they can form a condensed phase that’s biologically distinct from a more diffuse phase of biomolecules.
These remarkably simple drivers suggest that the phenomenon could be widespread in biology.
“We were surprised that it worked out in the end without requiring additional special circumstances, such as specific binding interactions,” said Feig, whose team revealed the mechanisms with computer simulations. Lapidus’s group then put those findings to the test with experiments.
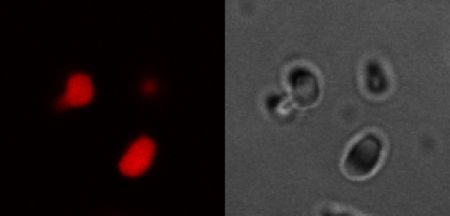
“To be honest, I was very skeptical this would work at all. I pushed pretty hard on Michael and Bercem to understand the results before I was willing to measure something.,” Lapidus said, referring to Bercem Dutagaci, the report’s lead author.
Dutagaci helped guide the project as a postdoctoral researcher in Feig’s group. She has since joined the faculty of the University of California, Merced.
“Phase separation is a key mechanism for forming membrane-less organelles, which are responsible for a number of functions in cells,” Dutagaci said, including metabolism and DNA replication. “These are the processes that help cells live.”
Although the results on phase separation surprised the researchers, their rigorous computational and experimental testing upheld the findings.
“There are always doubts that simulations generate nothing but fantasies, but in the end, we could confirm the idea via experiments and convince ourselves that what we saw in the simulations was real,” Feig said. “Bercem’s persistence and all of the team’s efforts to get to the bottom of this story despite many restrictions in a pandemic year were truly heroic.”
Banner image: A simulation of biological macromolecules in a highly simplified model of a bacterial cytoplasm suggests a propensity to form different kinds of condensates based on charge complementarity inside biological cells. These remarkably simple drivers suggest that the phenomenon could be widespread in biology. Simulation: Bercem Dutagaci; Figure credit: Michael Feig