The enzyme that changed the world and an MSU professor's career
NOTE: This story is one of several that appear in the most recent issue of the College of Natural Science Department of Physiology newsletter. To read about other research, activities and events happening in the department, visit https://natsci.msu.edu/publications/physiology.aspx.

When Bruce Uhal, professor in MSU’s Department of Physiology, began investigating the role of angiotensin-converting enzyme-2 (ACE-2) in lung injury and repair 16 years ago, he never imagined it would bring the world to a halt. ACE-2 is the main receptor, or docking molecule, for the SARS-CoV-2 virus that causes COVID-19 and led to half a million deaths in the United States.
“Starting in February 2020, I got a lot of calls and voicemails from people all over the world wanting to know how ACE-2 is controlled in the lungs and other organs,” Uhal said.
More than twenty years ago, while directing the Cardiovascular Research Institute at Michael Reese Hospital-UIC, Uhal’s lab showed that the peptide angiotensin II is involved in lung injury and repair. Unchecked angiotensin can cause hypertension, tissue fibrosis and epithelial cell death. After joining MSU in 2000, his lab was the first to describe how ACE-2, which degrades angiotensin II, protects the lungs from the injurious effects of this peptide.
When SARS emerged back in 2003, virologists and biochemists confirmed the enzyme that serves as an essential protective protein in the lungs was also the coronavirus’s preferred entry point for infection.
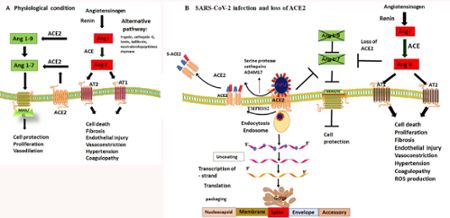
Then SARS-CoV-2 began surging in 2020, and ACE-2 was quickly found to be the main receptor for this coronavirus as well. At the time, Uhal was on the brink of retirement, but with so many lives at stake he instead switched to COVID-19 research, working first with Yong-Hui Zheng, professor in MSU’s Department of Microbiology and Molecular Genetics.
Soon afterward, Uhal joined an international group of scientists from India, Europe and the United States—the Self-Assembled Covid Research and Education Directive, or SACRED consortium.
The group of computational biologists, mathematicians, vaccine experts, clinicians and basic scientists published a flurry of papers in 2020. The consortium analyzed publicly available genetic sequences of SARS-CoV-2 and ACE-2 and used protein simulations that predicted future mutations, analyzed the effects of variants already emerging and generated a possible species flow of the virus. Their work is helping to establish the origin of SARS-CoV-2 and how it may have ultimately infected humans.
“The ACE-2 protein sequence differs across species, which has consequences for how tightly the virus can bind to it,” Uhal explained. Establishing the phylogeny, or evolutionary path of the virus, requires hundreds if not thousands of samples for sequencing. In the rush to solve this problem, the viral origin theories published so far were based on only a handful of samples, which is statistically inadequate.
Because the animal origin, or “zoonotic transfer” theory, of SARS-CoV-2 is based on insufficient sampling, the consortium has called for additional field samples of coronaviruses to be collected from bats, pangolins and civets across southeast Asia.
“In addition, we predicted a possible transmission flow from animals to humans and are now concerned that some animals might hold reservoirs of virus, including some household pets, based on their ACE-2 sequence,” Uhal said. Early in the pandemic, for example, Spain was forced to cull almost its entire mink industry due to coronavirus infections.
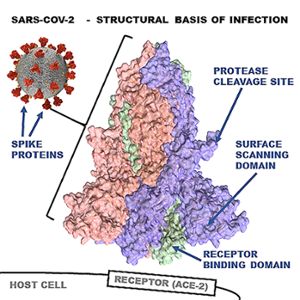
Since the pandemic started, very few mutations have occurred in the viral spike protein receptor binding domain, or RBD. According to the consortium, this suggests the protein has reached optimal binding affinity for ACE-2. This may explain why SARS-CoV-2 is the first coronavirus to reach pandemic levels and is key to Uhal’s successful collaborations with drug companies on the cutting-edge of COVID-19 therapeutics.
“ACE-2 abundance is highest in the upper nose and becomes less abundant further down the nasal passages and lungs,” Uhal explained. For this reason, it is now believed that we are infected through our noses first, and the virus then migrates down into the lungs. “Therefore, it should be theoretically possible to design decoy molecules based on the ACE-2 molecular structure, apply them as a nasal spray and ‘mop up’ the virus to prevent it from getting into the body.”
“It's a crazy, fascinating and critical task for scientists all over the world to keep up with this virus,” Uhal added. “I never imagined that I would end up studying a virus, but it became clear that what I’ve learned about ACE-2 is critical to helping the world figure out how to deal with SARS-CoV-2 and the other coronaviruses that will appear down the road. Unfortunately, I think we can count on more novel coronaviruses appearing in the future. If we learn only one thing from this pandemic, it should be that we had better be prepared.”
Banner image: When MSU physiology professor Bruce Uhal began investigating the role of angiotensin-converting enzyme-2 (ACE-2) in lung injury and repair 16 years ago, he never imagined it would bring the world to a halt. ACE-2 is the main receptor, or docking molecule, for the SARS-CoV-2 virus that causes COVID-19. When SARS-CoV-2 began surging in 2020, Uhal was on the brink of retirement, but with so many lives at stake he instead switched to COVID-19 research.