Cellular matchmaking: How viruses connect, infect bacterial partners

For the past year and a half, a particular coronavirus has been on everyone’s mind. But viruses of all kinds are always on the mind, and under the microscope, of Kristin Parent, J.K. Billman, Jr., M.D. Endowed Research Professor in the Michigan State University Department of Biochemistry and Molecular Biology (BMB).
Since joining MSU’s College of Natural Science in 2013, Parent has established herself as one of the nation’s leading experts of a class of viruses known as bacteriophage, or phage. Her pioneering research utilizes basic microbiology and cutting-edge cryo-microscopy to investigate, at the near atomic level, how phage use cell surface proteins to connect to, infect and reproduce inside some of the world’s deadliest gut bacteria—bacteria like Salmonella, E. coli and Shigella—destroying them in the process.
Starting June 1, Parent is lead investigator of a 5-year, $1.5 million National Institutes of Health (NIH) Maximizing Investigator’s Research Award (MIRA) that will continue to support her line of research developing an in-depth understanding of the structural and biochemical mechanics behind phage infection.
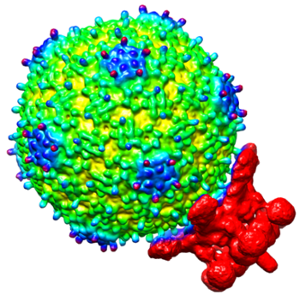
“In a previous R01 grant, my lab investigated the Sf6 phage that pairs with Shigella and learned a lot about how that model system has an innate ability to utilize more than one type of receptor protein to gain access to cells—this is unusual as some phages use only a single unique type of protein for this process,” Parent said. “With this grant, we can cast a much wider net to see how universal the pairing principles are, what is general and what is specific.”
Expanding the study of phage-host pairs from one to several hundred will allow Parent to delve deeper into the strategy behind how bacteriophage bind to a cell’s surface, transfer their genetic material across the cell membrane and use the bacteria’s own genetic equipment to replicate hundreds more phages.
“Viruses don’t all infect the same kind of cells,” explained Parent, who scoops some of the viruses for her research out of Michigan’s Red Cedar River that runs through campus. “It's like a matchmaking game, the virus has to have the right lock and key when it sticks to a cell, otherwise, they put genetic material into a cell they can’t infect and do not reproduce.”
Understanding how bacteriophage find the right bacterial partner is critical to advancing phage therapy—an alternative treatment to bacterial infections becoming increasingly resistant to antibiotics. So far, phage therapy has been a last option, but it could hold potential for patients suffering from Crohn’s disease, irritable bowel syndrome or an altered microbiome.
“Finding the right binding partner for the specific bacteria a patient is infected with is really challenging,” Parent said. “This research is a systematic way of testing the different outcomes of different phage-host pairings.”
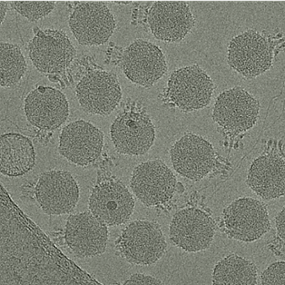
Besides traditional microbiology, the grant includes Michael McClelland, professor at the University of California, Irvine and an expert in the genetic sequencing and annotation of major pathogens including Salmonella enterica. His lab will utilize genetic manipulation to understand the evolution of phage infection, using complex transposon and single-gene deletion libraries key to propelling bacteriophage research forward.
MSU’s Cryo-EM facility, directed by Parent, will be utilized heavily to reconstruct three-dimensional pictures of phages and gain insight into the structural biology behind the phage ability to unlock cell surfaces and transfer their genetic material inside.
Because the bacteria they want to look at are too big for a microscope, Parent will collaborate with Ian Molineux, professor of molecular biosciences at the University of Texas at Austin, who engineers miniature versions of the cells that can be imaged more effectively.
The multidisciplinary approach also includes MSU’s Alex Dickson, BMB assistant professor. His computational molecular dynamics simulations will build on Parent’s cryo-microscopy images and provide new insights into phage infection mechanisms.

“One of the first things a phage sees when it encounters a bacterium are chains of sugar molecules attached to the bacterium’s cell wall,” explained Dickson, who holds a joint appointment in the Department of Computational Mathematics, Science and Engineering. “We know that the interactions between the phage’s spike protein and the sugars are crucial, but they’re too fast and too small to observe experimentally. My lab will use molecular simulations of the spike-sugar interactions to figure out how phages are able to infect some bacteria but not others.”
With the NIH funds secured, Parent is ready to dive deep into the surface interaction between phage and their not-so-lucky host bacteria.
“Because of Covid, women in academia have been hit particularly hard, so it’s come at a good time,” Parent said. “I’m grateful for the MIRA award that will provide long-term funding into the future of this research.”
Banner image: Research in the Parent lab utilizes basic microbiology, such as plating, and cutting-edge cryo-microscopy to investigate how bacteriophage (shown here attached to a bacterial cell wall (left) and in a petri dish (right)), use cell surface proteins to connect to, infect and reproduce inside some of the world’s deadliest gut bacteria. Credit: Emily Brown, https://commons.wikimedia.org/wiki/File:Bacteriophages_at_work.jpg