MSU researcher receives NIH grant to explore how cell signaling could lead to disease treatments
How do an organism’s cells respond to environmental changes or external stress at a molecular level? Scientists have learned that living cells respond to external changes by prompting adaptive phenotypes through a class of signaling molecules, cyclic di-nucleotides, or cdN, that connects environmental sensing to a cell’s internal regulatory networks and their phenotypic output.

Called second messenger signaling molecules because they relay signals received via cell-surface receptors (the first messengers), cdNs trigger key signaling changes at the cellular level, including proliferation, adaptation, survival and cell death. In humans, cdNs are a key regulator of the immune system that induces beneficial inflammatory responses to viral infection and cancer.
Despite cdNs important functions across all aspects of life, they have only been studied in bacteria for about 15 years and in multicellular organisms for just a few, leaving many unexplored questions about cdNs.
Chris Waters, a professor in the Department of Microbiology and Molecular Genetics, plans to address key questions about cdNs using a five-year, $2.62 million National Institutes of Health R35 Maximizing Investigators' Research Award (NIH MIRA).
“Recent evidence, including from my own laboratory, suggests that there is more to uncover and understand about the diversity of cdNs,” Waters said. “We do not know the phenotypes [traits] regulated by many cdNs or how they enhance adaptation and fitness. Thus, we are only just beginning to appreciate the importance of cdNs and the molecular mechanisms that enable them to function as transmitters of environmental sensing to phenotypic output, with more questions to answer.”
With this NIH award, Waters and his lab plan to explore questions on the diversity of cdN signaling systems, the environmental signals that regulate their synthesis and degradation, the molecular mechanisms that sense and respond to them, the phenotypes they regulate, and the adaptive benefit of such signaling systems. Since cdNs are critical for bacteria to cause disease as well as immune regulation in humans, understanding how they function can lead to new strategies to manipulate these systems for therapeutic treatments.
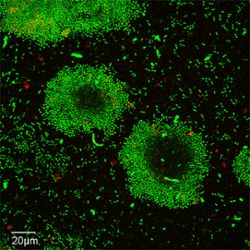
Waters’s lab has studied cdN signaling since its inception and has made fundamental contributions to the field, elucidating the mechanisms by which the cdN cyclic di-GMP helps the bacterial pathogen Vibrio cholerae—which causes cholera—persist in the environment and cause disease in humans. He and his lab have also expanded understanding of the traits controlled by cyclic di-GMP, including DNA repair, stress responses and control of cell shape.
These findings can be used to identify novel chemical compounds that interfere with cdN signaling as a potential treatment for disease caused by bacteria or beneficial manipulation of the immune system to treat infections or cancer.
“C-di-GMP presents a new target for the development of antimicrobial strategies because it controls both biofilm formation and virulence factor expression in numerous bacterial pathogens,” Waters said.
Banner image: Waters’s lab has studied cdN signaling since its inception and has made fundamental contributions to the field, elucidating the mechanisms by which the cdN cyclic di-GMP helps the bacterial pathogen Vibrio cholerae—which causes cholera (pictured above)—persist in the environment and cause disease in humans. Credit: Geoff Severin